Cyprus Natural Analogue Project (CNAP) - evidence for long-term, limited reactivity of bentonite in low alkali cement leachates
Bentonite is an important component in many designs for radioactive waste repositories. The plasticity, swelling capacity, colloid filtration, low hydraulic conductivity, high retardation of key radionuclides and stability in relevant geological environments all make bentonite an ideal barrier/buffer material in the engineered barrier system (EBS). However, bentonite is unstable in higher pH environments and this is a potential problem for repository designs which mix cement and concrete with bentonite barriers. The alkaline (initial pH~13) leachates from the cement are expected to degrade the bentonite, with it potentially losing some or all of its favourable properties. This has driven recent interest in low alkali cements, because the pH of the leachate is somewhat lower than standard OPC (Ordinary Portland Cement), lying around pH 10-11. It is hoped that this lower pH will reduce bentonite reaction (cf. Figure 1), so allowing the use of low alkali cements in close proximity with bentonite.
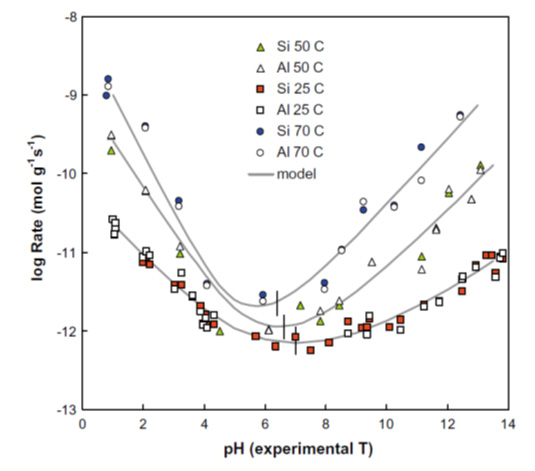
Figure 1: pH dependence of aluminosilicate reaction rates (Rozalén et al., 2009)
Assessing the long-term stability of bentonite in contact with such alkaline fluids under conditions representative of a deep geological repository requires complementary laboratory, modelling and in situ studies (see Alexander et al., 2015, in the bentonite folder in the library). In particular, to build a robust safety case, it is important to have supporting natural analogue data to confirm understanding of the likely long-term performance of bentonite.
Natural analogue (NA) studies could (see Alexander et al., 2014 in the bentonite folder in the library):
- Provide information on reaction rates, the products of such alteration and their safety-relevance to the performance of the engineered barrier system
- Allow testing of current models and databases used to assess such reaction
- Provide input to a range of supporting documents for safety cases
There are a number of locations worldwide where an appropriate natural analogue might be found, including Oman, California, Bosnia, Papua New Guinea and the Philippines (see Alexander et al., 2008 in the bentonite folder in the library for details). However, based on a multi-attribute analysis, considering factors such as probability of finding suitable locations with alkaline groundwaters and bentonite together, relevance to European national radioactive waste disposal programmes and logistics, Cyprus (Figure 2) was finally chosen as the focus for this particular study.
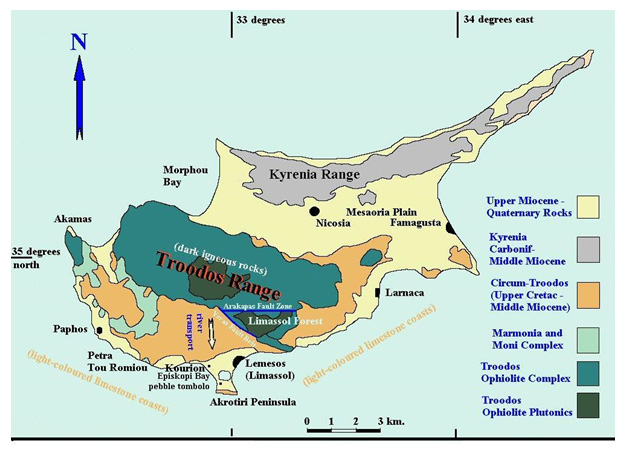
Figure 2: overview of the geology of Cyprus (West, 2007)
Here, the natural bentonites (as analogues of the industrial bentonites in the repository EBS) from the Parsata area of Cyprus have been examined (Alexander et al., 2014 in the bentonite folder in the library) for evidence of reaction with the underlying, naturally alkaline, groundwaters (as analogues of low alkali cement leachates) which are produced by reaction with a range of basic and ultrabasic rocks in the Troodos ophiolite (Figures 2 and 3). Two independent lines of evidence have been examined which suggest that the groundwater system has been running, at least intermittently, for some 105-106 a.
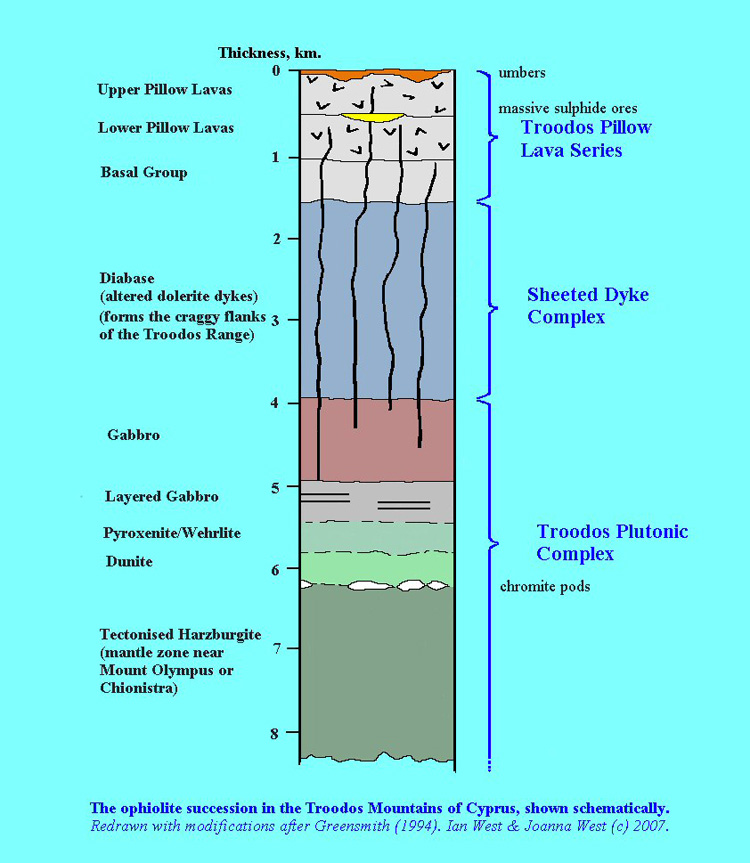
Figure 3: Schematic lithographic section of the Troodos ophiolite (West, 2007). The ‘umbers’ at the top of the sequence include bentonite analogues
There are clear signs of cation exchange, between the groundwaters and the bentonite, up to several metres from the base of the bentonite, although this appears to have had no appreciable impact on swelling pressures etc of the bentonite. A high pH ‘front’ would appear to have penetrated deeper into the bentonite analogue (as predicted in the past by coupled transport/reaction codes) and is not confined to the vicinity of the base of the bentonite. This front presumably propagated by diffusive transport of OH and Ca from the alkali groundwaters into the bentonite analogue. For at least one sample, the high Ca and Mg occupancy of the exchangeable cation sites coincides with the fact that this sample shows indications (from natural decay series data) of continuous reaction over the last ca. 0.8 x 105 a, suggesting intermittent and/or slow processes occurring here.
There are also indications (Milodowski et al, 2016) of very limited rock-water reaction at the very base (and along some fracture faces) in the bentonite which are thought to be the product of long-term reaction with the underlying alkali groundwaters (see Figure 4). However, any reaction has limited spatial reach (less than 1-2 mm from the base of the bentonite or fracture face), with the total volume of bentonite altered (from montmorillonite to palygorskite, an Mg-rich phyllosilicate) being significantly less than 1% of the total mass present.
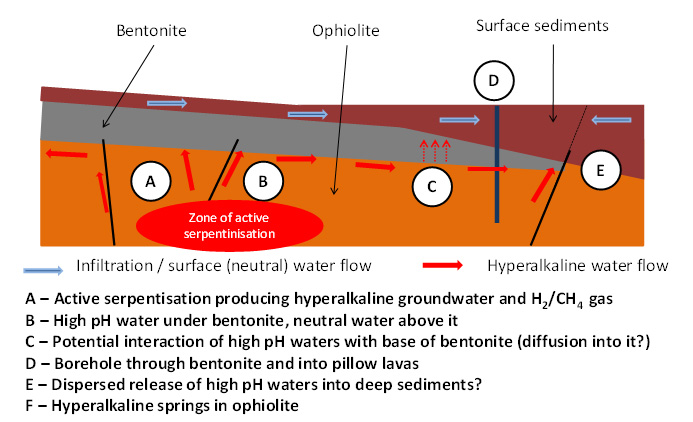
Figure 4. The natural system in Cyprus. Alkaline groundwaters could react with bentonite wherever they meet (Milodowski et al., 2016)
These results are supported by two independent lines of evidence : first, the presence of palygorskite is in accord with the more common occurances of this phase in natural systems where it is commonly formed by pedogenic processes and is found as an authigenic mineral under evaporative conditions, often associated with calcrete formation, in arid environment soils and palaeosols (e.g. Galan and Singer, 2011). It is also formed in highly saline, playa and alkaline lake sediments (e.g. Bristow and Milliken, 2011). Palygorskite can also precipitate directly from groundwaters with high Mg and Si activities (e.g. Hojati et al., 2012), but the textural relations observed in the Parsata samples would appear to rule this out here.
Second, recently published data from a medium-term (ca. 15a) laboratory experiment which indicates that, as the cement leachate plume evolves to lower alkalinity, an Mg-rich palygorskite or sepiolite appears to replace early reaction products such as CSH, ettringite and possibly apophyllite that originally replaced clay mineral or other phyllosilicates (see Rochelle et al., 1997 ; Moyce et al., 2014).
Although the precise mechanism for the smectite/palygorskite reaction is currently unclear, the results of the analyses presented here (i.e. focussed NA data from Cyprus, general natural system data from alkali environments and recent laboratory work) all indicate that, under appropriate conditions, palygorskite is a likely reaction product following reaction of bentonite in low alkali cement leachates (see also Milodowski et al., 2016, for discussion).
That there has been very limited alteration of the bentonite over a period of 105-106 a in Cyprus tends to indicate that any long-term bentonite reaction in low alkali cement leachates in a repository could be minimal. However, this may also be attributed, at least in part, to ‘self armouring’ of the bentonite as it naturally swells when encountering water. This emphasises the fact that any similar NA study of bentonite longevity must also take into account physical scales so that the study conditions approach those of a repository as closely as possible as only then will the latent physico-chemical behaviour of the bentonite be observed (see also Reijonen & Alexander, 2015, for discussion).
Acknowledgements
The financial support of the three Project Partners, NDA-RWMD (UK), Posiva (Finland) and SKB (Sweden) is gratefully acknowledged.
References
Bristow, T.F & Milliken, R.E. (2011) : Terrestrial perspective on authigenic clay mineral production in ancient Martian lakes. Clay and Clay Mins. 59, 339 - 358.
Galan, E. & Singer, A. (eds) (2011): Developments in palygorskite – sepiolite research: a new outlook on these materials. Developments in clay sciences 3, Elsevier, Amsterdam, The Netherlands.
Hojati, S., Khademi, H. & Cano, A.F. (2010): Palygorskite formation under the influence of groundwater in central Iranian solis. Anadolu Tarım Bilim. Derg., 25(S-1), 34 – 41.
Milodowski, A.E., Norris, S. & Alexander, W.R. (2016). Minimal alteration of montmorillonite following long-term reaction in natural alkali solutions: implications for geological disposal of radioactive waste. Appl. Geochem. 66, 184-197.
Moyce, E.B.A, Rochelle, C.A. et al. (2014). Rock alteration in alkaline cement waters over 15 years and its relevance to the geological disposal of nuclear waste. Appl. Geochem. 50, 91-105.
Reijonen, H.M. and Alexander, W.R. (2015). Bentonite analogue research related to geological disposal of radioactive waste – current status and future outlook. Swiss Journal of Geosciences 108, 101-110. DOI 10.1007/s00015-015-0185-0
Rochelle, C.A., Pearce, J.M., Bateman, K., Coombs, P. & Wetton, P.D. 1997. The evaluation of chemical mass transfer in the disturbed zone of a deep geological disposal facility for radioactive wastes. X: Interaction between synthetic cement porefluids and BVG: Observations from experiments of 4, 9 and 15 months duration. British Geological Survey (BGS), Fluid Processes and Waste Management Group Report, WE/97/16C, 79p. BGS, Keyworth, UK.
Rozalen, M., Huertas, F.J. and Brady, P.V. (2009). Experimental study of the effect of pH and temperature on the kinetics of montmorillonite dissolution. Geochimica et Cosmochimica Acta 73, 3752-3766.
West, I.M. (2007). Geology of the Salt Lake and Coast of the Akrotiri Peninsula, Cyprus. National Oceanography Centre, Southampton, UK. Internet site. http://www.soton.ac.uk/~imw/Cyprus-Akrotiri-Lake-Coast.htm. Version: 16 January 2007.

